Prevalence of Expanded Spectrum Beta-lactamase
Introduction
Gram-negative bacteria pose the biggest hazard to human health because they are resistant to a wide range of antibiotics (Egbule and Odih, 2020). Bacterial diseases at KRRH are often treated with -Lactam medicines. On the other hand, Gram-negative bacteria develop enzymes called -lactamases, which operate as a critical resistance against -lactam antibiotics (Hijazi et al., 2016). According to Bertrand (2003), ESBLs are the kind of -lactamase catalysts generated by some Gram-negative bacteria of the Enterobacteriaceae family. Afiridi and Farooqi (2012) assert that Aztreonam, cephalosporin drugs, and hydrolysis of penicillin confer resistance to ESBL enzymes generated by mobile genetic. Even more worrisome is that these genes encode resistance to a wide range of antibiotics, including tetracyclines, fluoroquinolones, penicillin, and cephalosporins. The limited treatment options for treating ESBL-producing species of the Enterobacteriaceae, which leads to poor clinical outcomes and their increased propensity to trigger hospital-acquired diseases, have drawn attention in hospital settings (Van de Bunt et al., 2019). As a result, hospitalized individuals become reservoirs for these microorganisms. Many different species of bacteria in the Pseudomonadaceae and Enterobacteriaceae families have been found to generate ESBLs. However, the most prevalent are Proteus sp, pneumonia, and E. coli, K. A beta-lactam antibiotic therapy for ESBL-producing bacteria is unlikely because of the danger of treatment failure and increased infectiousness. Early diagnosis of these germs is critical to prevent and manage nosocomial epidemics in hospitals (Voor in’t holt, 2016).
Global public health has been plagued by antimicrobial resistance (AMR) produced by bacteria, viruses, fungi, and parasites. A wide variety of illnesses, including pneumonia, wound infections, bloodstream infections, and urinary tract infections, are at risk from the spread of antibiotic-resistant bacterial strains, which pose a substantial threat to successful preventive and control methods (Ouchar Mahamat et al., 2019). Global health is at risk from ESBL-PE, especially Gram-negative bacteria like Klebsiella pneumoniae and E. coli which have developed a resistant strain pattern. As a result of lax medication regulation and management, antibacterial medicines are routinely misused and overused by people and animals alike (Braide et al., 2018). As a result of these actions, resistant bacterial strains are more likely to spread across the community and into healthcare facilities, decreasing the effectiveness of treatment. As a result, drug-resistant microbes variants can proliferate and spread wherever infection control practices mechanisms are insufficient. Diseases caused by ESBL-producing Gram-negative bacteria pose significant difficulties to the health sector involved in diagnostics, treatments, disease prevention and management, and producing a novel antimicrobial drug to combat AMR’s catastrophic consequences (Kannaiyan et al., 2018).
Preventative measures, difficult therapy owing to multi-resistant ESBLs, and no modern diagnostic laboratory all contribute to the poor healthcare quality to battle ESBLs. As a consequence, nosocomial and community-acquired illnesses continue to be a major focus of clinical recommendations and research investigations (Bertrand, 2003).
The most common medicines used for infections caused by ESBL-PE are beta-lactam antibiotics, such as carbapenems, monobactams, cephalosporins, and extended-spectrum penicillins. ESBL-PE continues to be a leading cause of community-acquired and hospital infections globally, despite the widespread use of beta-lactam antibiotics. (Kiro et al., 2021) Today, ESBL-PE is a leading cause of infection outbreaks, creating difficult infection control difficulties and compromising a wide spectrum of clinical results. Members of the Enterobacteriaceae family with plasmid-mediated ESBL resistance are also readily transmissible, as is this resistance. There will be fewer antimicrobial drugs available to treat illnesses if this situation persists. Global public health has been plagued by antimicrobial resistance (AMR) produced by bacteria, viruses, fungi, and parasites (Moradi et al., 2021). A wide variety of illnesses, including urinary tract infections, bloodstream infections, wound infections, and pneumonia, are at risk from the spread of antibiotic-resistant bacterial strains, which pose a substantial threat to successful preventive and control methods. Global health is at risk from ESBL-PE, especially Gram-negative bacteria like E. coli and Klebsiella pneumoniae, which have developed a resistant strains pattern. ESBL-PE (Herindrainy et al., 2021).
As a result of lax medication regulation and management, antibacterial medicines are routinely misused and overused by people and animals alike. As a result of these actions, resistant bacterial strains are more likely to spread across the community and into healthcare facilities, decreasing the effectiveness of treatment (Hawkey, 2008). As a result, drug-resistant microbes variants can proliferate and spread wherever infection control practices mechanisms are insufficient. Diseases caused by ESBL-producing Gram-negative bacteria pose significant difficulties to the health sector involved in diagnostics, treatments, disease prevention and management, and producing a novel antimicrobial drug to combat AMR’s catastrophic consequences.’ Preventative measures, complex therapy owing to multi-resistant ESBLs, and no modern diagnostic laboratory contribute to the poor healthcare quality to battle ESBLs (Andrew, 2017).
The most common medicines used for infections caused by ESBL-PE are beta-lactam antibiotics, such as extended-spectrum penicillins, cephalosporins, monobactams, and carbapenems. ESBL-PE continues to be a leading cause of hospital and community-acquired infections globally, despite the widespread use of beta-lactam antibiotics. Today, ESBL-PE is a leading cause of infection outbreaks, creating difficult infection control difficulties and compromising a wide spectrum of clinical outcomes (Moradi et al., 2021). Members of the Enterobacteriaceae family with plasmid-mediated ESBL resistance are also readily transmissible, as is this resistance. There will be fewer antimicrobial drugs available to treat illnesses if this situation persists. Since many patients now want carbapenems, the screening of infections immune to carbapenems has accelerated. For these reasons, as well as higher medical expenditures, treatment failures, extended hospital stays, and an increase in death rates, the alarmingly high rates of AMR mainly caused by Gram-negative bacteria are cause for worry (Ouchar Mahamat et al. (2019). Despite this, there is no systematic investigation of the whole Birmingham population to determine the prevalence of ESBL. ESBL-PE surveys in the local context are critical for identifying local clinical and epidemiological data gaps. In addition, local epidemiological data allow health specialists to estimate the costs of implementing breakthrough measures for infection control practices to reduce community-acquired and nosocomial infection related to ESBL-producing pathogens.-producing. Consequently, this research attempted to synthesize the various investigations on ESBL-producing organisms and estimate the incidence of ESBL-PE in Birmingham by meta-analytical approaches.
Methods
Located in the West Midlands region of England, Birmingham is a metropolitan borough and a city. At over 1.1 million people inside the city’s boundaries and 2.9 million people in the metropolitan environment, England’s second-largest urban area and the United Kingdom’s third-largest metropolitan region. Using normal bacteriological methods, any laboratory-based research that addresses the direct result of interest in light of the idea of the predominance of extended-spectrum -lactamase-producing Enterobacterialee were comprehensively analyzed. As a result, a systematic review and meta-analysis research was conducted, to sum up, the prevalence of bacterial isolates collected from diverse human specimens reported at any period without date constraint.
For grey and peer review literature, no date limits were used to a comprehensive literature search technique aimed at systematic review identified prevalence of extended-spectrum -lactamase-producing Enterobacteriaceae. As a first step, the author conducted a thorough search of electronic databases such as Scopus, Cochrane Library, PubMed, and MEDLINE to identify possibly relevant literature. This study picked the search terms and the appropriate sources for the review. Consequently, the MeSH (Medical Subject Heading) search approach produced keywords. In addition, the study adjusted the wild cards (“”) and Boolean operators (NOT, OR, AND) depending on the result metrics. “Prevalence” OR “epidemiology of ESBL” AND “Birmingham”, “Extended-spectrum beta-lactamase-producing Enterobacteriaceae,” “ESBL infections,” “Multidrug-resistant bacteria,” and “Extended-spectrum beta-lactamase-producing isolates” were all used in the search strategy to get relevant results. The writers separately evaluated the article’s entire text, lacking or inadequate to get further details. Disputes were settled by conversation until a consensus was formed. Then, each article’s data was entered into a spreadsheet and analyzed. There was a thorough review of each study’s references and data sets to verify that no overlapping data was provided. Ultimately, 12 publications were qualified for inclusion in the research.
Empirical Review
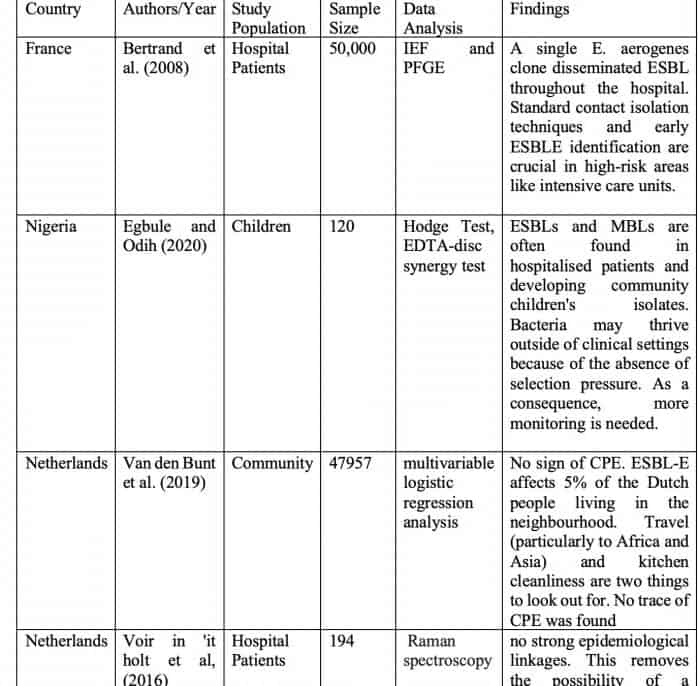
Bertrand et al. (2008). examined enterobacteriaceae that produce extended-spectrum beta-lactamase (ESBLE) (2008). The occurrence of ESBLs in Enterobacteriaceae was examined for two years. PFGE and IEF were also utilized to define ESBLs and investigate the epidemiological connection between EBLSE and PFGE. Seventy patients were diagnosed with EBLSE (0.095 per 1000 patient-days) during this period. This pathogen, Enterobacter aerogenes, was the major vector for ESBLE dissemination. 59.5 percent of the ESBLE were E. aerogenes, and 21.9 percent were E. aerogenes plasmid-transferred. According to IEF and PFGE results, a single E. aerogenes clone disseminated ESBL throughout the hospital. Standard contact isolation techniques and early ESBLE identification are crucial in high-risk areas like intensive care units.
This study by Egbule and Odih (2020) found MBLs and ESBLs in isolated bacteria from community and hospital settings in Delta State, Nigeria. The samples were examined microbiologically to identify 84 isolates from patients and the general population. The carbapenemase was detected using a modified Hodge test. The EDTA-disc synergy test revealed MBL generation in 26.2% of isolates. The DDST found ESBLs in 75.7% of Klebsiella pneumonia, 37.7% of P. aeruginosa, and 36.7% of E. coli samples. MBL and ESBL co-produced in 31.8 percent of isolates. The study drew two frightening results. ESBLs and MBLs are often found in hospitalized patients and developing community children’s isolates. Bacteria may thrive outside of clinical settings because of the absence of selection pressure. As a consequence, more monitoring is needed.
Van den Bunt et al. (2019) investigated why CPE and ESBL-producing Enterobacteriaceae are not more common in the general population. The Dutch research looked at intestinal ESBL-E and CPE carriage frequency and the factors that led to that frequency. ESBL-E was characterized. Between 2014 and 2016, 2,000 feces samples were collected monthly and analyzed for ESBL-E. The first 1,758 samples had CPE. Multiple logistic regression was performed to detect ESBL-E risk factors. ESBL-E isolates were whole-genome sequenced. The research gathered feces from 4,177 of the 47,957 persons who agreed to participate. ESBL-E was detected in 186 persons (4.5%), giving a prevalence estimate of 5.0 percent (95% CI: 3.4–6.6%). Born outside the Netherlands (OR: 1.99; 95 percent CI: 1.16-4.54), eating out more than 20 times per year (OR: 1.70; 95 percent CI: 1.04-2.76), taking antibiotics within the last six months (OR: 2.05; 95 percent CI: 1.05-4.03), and swimming in the sea or ocean within the last year (OR: 1.63; 95 percent CI: 1.11-2.39). (47.5 percent). 84 (44.4%) of the 189 ESBL-E isolates carried blaCTX-M-15. The most prevalent ST131 (42/178 isolates; 23.6%) was found among the 70 Escherichia coli STs. They were found with ST131 and blaCTX-M-27 and ST88 with blaCTX-M-1. No sign of CPE. ESBL-E affects 5% of the Dutch people living in the neighborhood. Travel (particularly to Africa and Asia) and kitchen cleanliness are two things to look out for. No trace of CPE was found.
Voir in’it holt. (2016) used Raman spectroscopy and HiMLST to identify recent E. coli transmission events. The study was conducted in Rotterdam from January to December 2019 at Erasmus University Medical Center. The isolates were classified using HiMLST and Raman spectroscopy. A genetic cluster consisted of two or more patients with similar isolates. Researchers used epidemiological relatedness parameters to estimate illness transmission in hospitals. HiMLST typed 112 patient strains, whereas Raman spectroscopy typed 194. There were 16 Raman clusters and 10 HiMLST clusters. The only thing identified was the lack of transmission associated with medical therapy. The analysis revealed eight clusters (n = 34 patients) and 78 non-cluster isolates (n = 78). But no healthcare transmissions were found in these eight clusters. Using HiMLST and Raman spectroscopy to find genetic clusters revealed no strong epidemiological linkages. This removes the possibility of a healthcare-related epidemic. AHIMLST or Raman spectroscopy does not help us detect E. coli healthcare transmission episodes, according to the results.
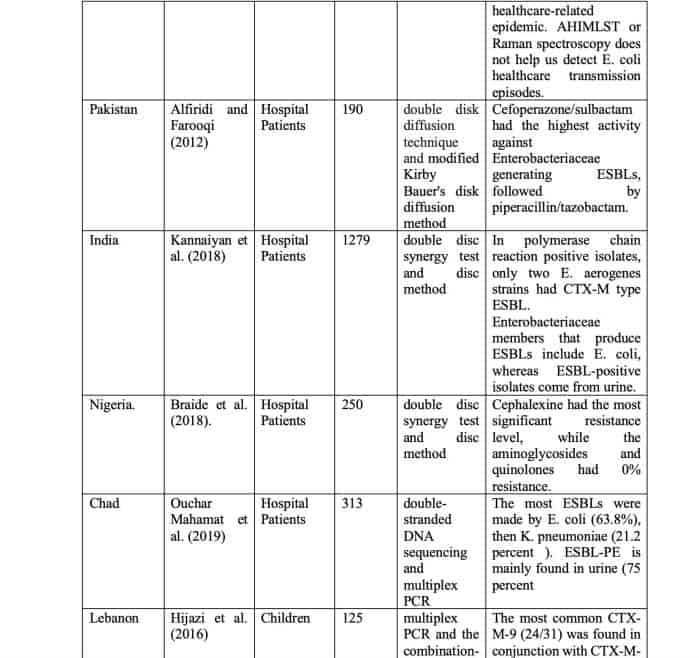
ESBL-producing Enterobacteriaceae susceptibility patterns for beta-lactam beta-lactamase inhibitor combinations in urine isolates were examined by Alfiridi and Farooqi (2012). Observational research was employed to collect the data. From February through October 2008, the study took place at Karachi’s Ziauddin University. The study comprised 190 non-duplicate ESBL-producing Enterobacteriaceae isolates from in-patient urine testing. Inpatient samples that did not develop ESBL were also ruled out. The ESBL was discovered utilizing double-disk diffusion. A modified Kirby Bauer’s disk diffusion method investigated antibiotic susceptibility. The statistical analysis was done using SPSS 10. Only 88 (46.31 percent) of the isolates examined were susceptible, whereas six (3.15 percent) were resistant to all three antibiotic combinations; amoxicillin/clavulanic acid (44.31%), piperacillin/tazobactam (92.10), and Sulbactam/cefoperazone (95.26%). The most effective ESBL-fighting combination was Cefoperazone/Sulbactam, followed by Piperacillin/Tazobactam. Hospital antibiotic policies should be reviewed routinely to reduce the use of extended-spectrum cephalosporins and replace them with beta-lactam beta-lactamase inhibitor combinations.
Kannaiyan et al. (2018) investigated ESBL producers in clinical samples from questionable individuals. 154 stool, 207 pus, and 918 urine samples were obtained; 465 were extracted and tested for ESBL producers using a double-disc synergetic test and mixed disc approach (Klebsiella pneumoniae, Enterobacter aerogenes, and Escherichia coli). Out of 465 culture-positive isolates examined, 130 (E. coli 93 [29.06 percent], K. pneumonia 2 [7.69 percent] and E. aerogenes 35 [29.41 percent] produced ESBL. Among the three Enterobacteriaceae groups, E. coli 93 (29.06%) produced the highest ESBLs, followed by E. aerogenes 35 (29.41%) and K. pneumoniae 2. (7.69 percent ). Urine (n=111) contained the most ESBL producers, followed by pus (n=14) and stool (n=5). All ESBL-producing isolates were tested with ten different antibiotics. They were the most common ESBL manufacturers. A total of 130 ESBLs were identified using 15 strains (E. coli, E. aerogenes, and K. pneumoniae). In polymerase chain reaction positive isolates, only two E. aerogenes strains had CTX-M type ESBL. Enterobacteriaceae members that produce ESBLs include E. coli, whereas ESBL-positive isolates come from urine. The best approach to differentiate ESBL types was by genotype.
Pseudomonas and Escherichia bacteria that produce ESBLs were found at the Federal Medical Centre (FMC) Owerri, Imo State, Nigeria, by Braide et al. (2018). More than 200 E.coli (136) and Pseudomonas aureus (114) clinical isolates were employed in the investigation. The disc diffusion technique was used to investigate the sensitivity of isolates to antimicrobials. The double-disc synergy test was used to examine the ESBL phenotypes of Ceftazidime, Cefotaxime, Ceftriaxone, and Amoxicillin clavulanic acid in the bacterial samples. 114 of the 250 isolates examined were positive for ESBL production, with 66 (26.4%) E. coli and 48 (19.2%) P. aeruginosa making up most of the samples. Testing the antimicrobial sensitivity indicated that Cephalexine had the most significant resistance level, while the aminoglycosides and quinolones had 0% resistance. This indicates that the aminoglycosides and quinolones may be used to treat ESBL infections produced by these organisms. According to the research, the Federal Medical Centre (FMC) in Owerri, Imo State, Nigeria, seems to have a high frequency of ESBL-producing E. coli and P. aeruginosa.
Ouchar Mahamat et al. (2019) looked at the genetics of ESBL-PE in three N’Djamena hospitals. From 1713 patients in N’Djamena’s three main hospitals, 313 non-duplicate isolates have been found. T-FMS identified bacterial species using matrix-aided laser desorption ionization. Using disk diffusion on Müller-Hinton agar, the double-disc synergy test showed ESBL production and sensitivity to 28 antibiotics. The study identified the most prevalent ESBL resistance genes with double-strand DNA and multiplex PCR sequencing. Among the 313 isolates, 197 were Enterobacteriaceae. ESBL-PE affected 47.72 percent of individuals (94/197). (54.13%) It affected adults over 60. The most ESBLs were made by E. coli (63.8%), then K. pneumoniae (21.2 percent ). ESBL-PE is mainly found in urine (75 percent ). Three CTXM groups: 1 (96.7%), 9 (96.7%) and 9 (96.7 percent ). (4.1%) 86% of resistant isolates showed several ESBL genes. Non-lactam resistance was connected to ESBL production. In three main hospitals in Chad, 48 percent of clinical isolates contained ESBL-PE with CTX-M-1 group ESBLs.
Hijazi et al. (2016) investigated the incidence of ESBL-PE faeces carriage, antibiotic sensitivity patterns, and risk factors in Lebanese children. One hundred twenty-five healthy youngsters aged 1 to 5 years had rectal swabs collected. ESBLs were detected using multiplex PCR and the combination-disc technique. A survey on a child’s lifestyle and ESBL risk factors was shown. 31 out of 125 subjects (24.8%) had ESBL-PE. Meat and chicken intake were related to elevated ESBL-PE carriage, but not milk-based products (yogurt, cheese). Intimate hygiene behaviors influenced the carriage rate. The most prevalent bla genes were bla CTX-M and bla TEM, followed by bla SHV and bla TEM. The most common CTX-M-9 (24/31) was found in conjunction with CTX-M-15. The high prevalence of colonization in healthy youngsters with ESBL-PE was linked to frequent eating of animal products (meat or chicken). For the first time in Lebanon and the Middle East, there was data on the prevalence of ESBL-PE society carriage and the CTX-M-9 variant in this population.
Table 1: Summary of Empirical Findings
Discussion of Results
Using a systematic review, this study discovered phenotypic and genetic clusters of ESBL-producing E. coli, but no clinical links between patients were detected. Increasing worldwide travel, communication, and commerce is driving a rise in ESBL and other antibiotic resistance genes transmission. This global rise in ESBL genes implies actual treatment failures. Many dangerous urinary, gastrointestinal, and wound infections no longer respond to beta-lactam antibiotics. Antimicrobial resistance data from common infections is helpful in treatment selection. Clinicians should be aware of treatment failures associated with ESBL generating bacteria, especially gram-negative Enterobacteriaceae. The results verified the high prevalence of blaTEM and blaCTX-M genes in Enterobacteriaceae isolates from Khartoum hospitals. E. coli and K. pneumonia had the most significant antimicrobial resistance and the highest likelihood of possessing multiple genes. The multidrug-resistant profile is linked with the present genes. Thus, regular ESBL screening in clinical isolates is critical to preventing resistant isolates from spreading in health care settings. Our results corroborate previously published data from the area. However, all isolated and high have -Lactamase. Third-generation cephalosporins showed resistance. -lactamase-producing isolates must be identified and treated accordingly. These isolates cause numerous nosocomial outbreaks, deaths, and high hospital expenses owing to treatment failure. Treatment options for ESBL-producing bacteria are limited.
Carbapenems are the preferred therapy for ESBL-producing pathogens. These days, however, there are carbapenem-resistant isolates. Enterobacteriaceae are suited to genetic exchange. Resistance is attributed chiefly to transposable resistance genes mediated in most by plasmids. Different mobile genetic elements are blamed for encapsulating these genes from chromosomes of different bacterial species and shifting them horizontally across bacteria.
The carriage of several resistance genes on one plasmid helps the bacterial cell to reach multi-resistance in one move. The studies reviewed combines clinical and molecular epidemiology (genetic and phenotypic) data to discover healthcare-related transmission events in non-epidemic settings. Following the findings of the multidrug-resistant microbe recommendations, all E. coli and ESBL-producing should be investigated to identify and manage healthcare-related transmission occurrences. However, the specific definition of a ‘healthcare-related transmission event’ is unknown, as are the suggested typing procedures. Also, it is unclear how the findings should be understood. The addition of a beta-lactam beta-lactamase inhibitor combination (piperacillin/tazobactam) to the hospital formulary and restriction of third-generation cephalosporins (ceftazidime) was associated with a decrease in the percentage of ceftazidime resistant isolates as well as colonization and infection by ESBL producing E. coli or K. pneumoniae in patients admitted to the ESBL-producing organisms like UTIs may be treated with beta-lactams beta-lactamase inhibitors combinations like cefoperazone/sulbactam and piperacillin/tazobactam. They seem to inhibit the development of ESBL generating bacteria compared to third-generation cephalosporins (such as ceftriaxone or ceftazidime).
Conclusion
Cefoperazone/sulbactam demonstrated the most significant action against urine isolates of ESBL generating Enterobacteriaceae, preceded by piperacillin/tazobactam. The effectiveness of these combination drugs should be studied in clinical trials. Regularly evaluate hospital antibiotic policies to restrict the use of third-generation cephalosporins and replace them with potent beta-lactamase inhibitor mixtures to lessen selection pressure and prevent antimicrobial resistance. There is a need for more empirical results on the prevalence of expanded spectrum beta-lactamase in Birmingham.
References
Afridi FI, Farooqi BJ., 2012. The activity of beta-lactam beta-lactamase inhibitor combinations against extended-spectrum Beta-lactamase producing Enterobacteriaceae in urinary isolates. J Coll Physicians Surg Pak., 22(6):358-62. PMID: 22630093.
Andrew, B., Kagirita, A., & Bazira, J., 2017. Prevalence of Extended-Spectrum Beta-Lactamases-Producing Microorganisms in Patients
Admitted at KRRH, Southwestern Uganda. International Journal of Microbiology, 2017, 1–5. doi:10.1155/2017/3183076
Bertrand, X. (2003). Molecular epidemiology of Enterobacteriaceae producing extended-spectrum β-lactamase in a French university-affiliated hospital. International Journal of Antimicrobial Agents, 22(2), 128–133. doi:10.1016/s0924-8579(03)00098-0
Braide, W., Madu, L.C., Adeleye, S.A, Korie, M. C. & Akobondu, C.I., 2018. Prevalence of Extended Spectrum Beta-Lactamase Producing Escherichia coli and Pseudomonas aeruginosa Isolated from Clinical Samples. International Journal of Sciences 4(02):89-93
Egbule, O.S., Odih, E.E., 2020. Prevalence of Extended-Spectrum Beta-Lactamases (ESBLs) and Metallo-beta-lactamases (MBLs) Among Healthy and Hospitalised Children in Abraka and Eku Communities, Delta State, Nigeria. Nigerian Journal of Basic and Applied Science (June 2020), 28(1): 07-14
Hawkey PM. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin Microbiol Infect. 2008. Erratum in: Clin Microbiol InfectSuppl 1:159-65. DOI: 10.1111/j.1469-0691.2007.01855.x Suppl 5:21-4. PMID: 18154540.
Herindrainy P, Rabenandrasana MA, Andrianirina ZZ, Rakotoarimanana FM, Padget M, de Lauzanne A, Ndir A, Kermorvant-Duchemin E,
Garin B, Piola P, Collard J.M., 2018. Acquisition of extended-spectrum beta-lactamase-producing Enterobacteriaceae in neonates: a community-based cohort in Madagascar. PloS one 13(3):1–17
Hijazi, S.M., Fawzi, M.A., Ali, F.M. et al., 2016. Prevalence and characterization of extended-spectrum beta-lactamases producing Enterobacteriaceae in healthy children and associated risk factors. Ann Clin Microbiol Antimicrob 15, 3. https://doi.org/10.1186/s12941-016-0121-9
Kannaiyan, M., Meseret Abebe, G., Kanimozhi, C., Thambidurai, P., Ashokapuram Selvam, S., Vinodhini, R., & Suresh, M., 2018. Prevalence Of Extended-Spectrum Beta-Lactamase Producing Enterobacteriaceae Members Isolated From Clinically Suspected Patients. Asian Journal of Pharmaceutical and Clinical Research, 11(5), 364. doi:10.22159/ajpcr.2018.v11i5.19
Kiros, T., Workineh, L., Tegenaw, T., Tahir, E., Shewaneh, D. Debaka, B., 2021. Prevalence of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Ethiopia: A Systematic Review and Meta-Analysis”, International Journal of Microbiology, https://doi.org/10.1155/2021/6669778
Moradi, Y., Eshrati, B., Motevalian, S. A., Majidpour, A., & Baradaran, H. R., 2021. A systematic review and meta-analysis on the prevalence of Escherichia coli and extended-spectrum β-lactamase-producing Escherichia coli in pregnant women. Archives of Gynecology and Obstetrics, 303(2), 363–379. doi:10.1007/s00404-020-05903-w
Ouchar Mahamat, O., Lounnas, M., Hide, M., Dumont, Y., Tidjani, A., Kamougam, K., … Godreuil, S., 2019. High prevalence and characterization of extended-spectrum ß-lactamase producing Enterobacteriaceae in Chadian hospitals. BMC Infectious Diseases, 19(1). doi:10.1186/s12879-019-3838-1
van den Bunt Gerrita, van Pelt Wilfrid, Hidalgo Laura, Scharringa Jelle, de Greeff Sabine C., Schürch Anita C., Mughini-Gras Lapo, Bonten Marc J.M., Fluit Ad C.., 2019. Prevalence, risk factors, and genetic characterization of extended-spectrum beta-lactamase and carbapenemase-producing Enterobacteriaceae (ESBL-E and CPE): a community-based cross-sectional study, the Netherlands, 2014 to 2016. Euro Surveill, 24(41):pii=1800594. https://doi.org/10.2807/1560-7917.ES.2019.24.41.1800594
Voor in ‘t holt, A. F., Wattel, A. A., Boers, S. A., Jansen, R., Hays, J. P., Goessens, W. H. F., & Vos, M. C., 2016. Detection of Healthcare-Related Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Transmission Events Using Combined Genetic and Phenotypic Epidemiology. PLOS ONE, 11(7), e0160156. doi:10.1371/journal.pone.0160156
If you like this article, see others like it:
- Evaluating the Status of Schistosomiasis in Dabban, Lavun LGA, Niger State
- Assessment of Female Genital Mutilation in Nigeria
- Prevalence of Expanded Spectrum Beta-lactamase in Birmingham: A Systematic Review
- Effect of a Nurse-led Training Programme on Pressure Ulcer Prevention and Treatment Among Nurses in Two Teaching Hospitals
- Effects of COVID-19 on Energy Investment